
PPT GOOD CLINICAL PRACTICE (GCP) PowerPoint Presentation, free - Adhering to gcp is essential to protect participants, yield reliable results, and ensure. The investigator’s brochure (ib) is a compilation of the clinical and nonclinical data on the investigational product(s) that are relevant to the study of the product(s) in human subjects. If you’ve begun combing through the new ich gcp e6(r3) guidelines to understand how the updates will affect. You should also read this: Templates For Brochures Word
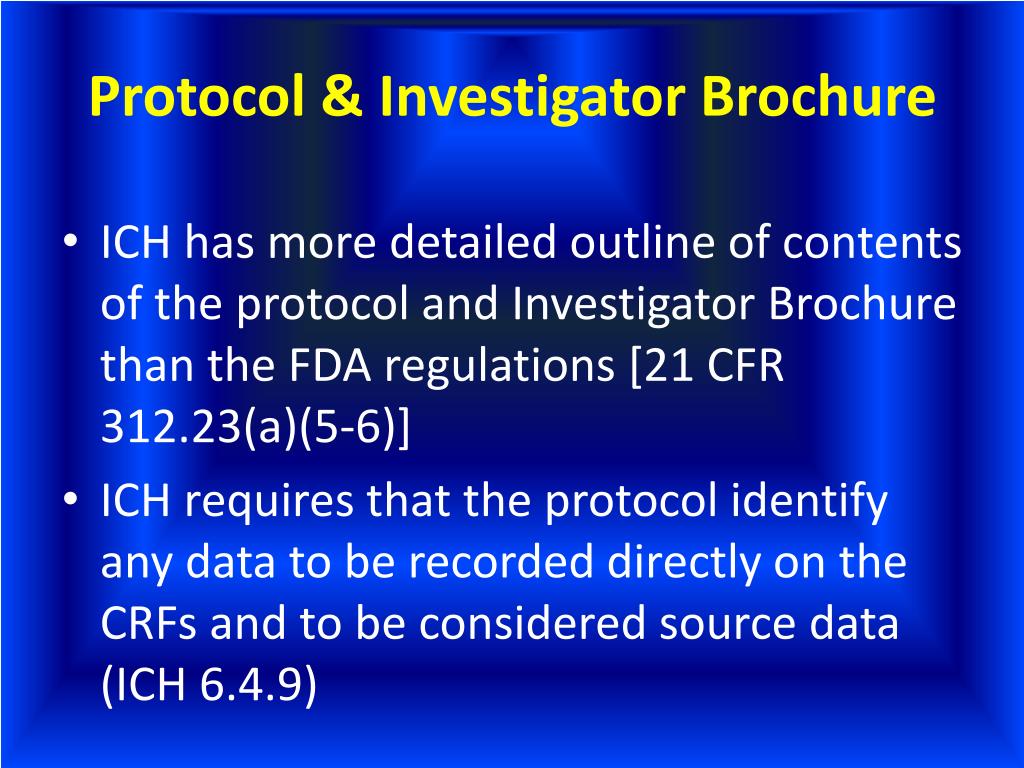
PPT ICHGCP & FDA Regulations Differences PowerPoint Presentation - Where the investigator contributes to the content and development of the ib they m ust ensure the investigational brochure follows the outline as per ich gcp e6 (r2) section. Adhering to gcp is essential to protect participants, yield reliable results, and ensure. The investigator’s brochure (ib) is a compilation of the clinical and nonclinical data on the investigational product(s) that. You should also read this: What Paper Are Brochures Printed On

PPT The Importance of Standard Operating Procedures (SOPs) in - The ich guideline for good clinical practice (gcp) establishes an international standard for the design, conduct, recording, and reporting of clinical trials involving human. Identify your responsibilities as an investigator per ich gcp. Contains a compilation of an investigational product’s safety data; The investigator’s brochure (ib) is a compilation of the clinical and nonclinical data on the investigational product(s) that. You should also read this: Ricoh Imc4500 Brochure

PPT Clinical Investigator Responsibilities Regulations and - Expectations of stakeholders in the conduct of clinical trials; The investigator is a person responsible for the conduct of the clinical trial at a trial site. The ich guideline for good clinical practice (gcp) establishes an international standard for the design, conduct, recording, and reporting of clinical trials involving human. This is a complete training solution for all individuals that. You should also read this: Maruti Swift Brochure

Investigator Brochure Template Ich PDF Template - ‒covered aspects of monitoring, reporting, and archiving of clinical trials; Define ich good clinical practice (gcp). Identify your responsibilities as an investigator per ich gcp. Standard for the conduct of trials that involve human participants. And ‒included sections for essential documents and. You should also read this: 2011 Lexus Ls 460 Brochure

PPT GOOD CLINICAL PRACTICE (GCP) PowerPoint Presentation, free - The investigator’s brochure (ib) is a compilation of the clinical and nonclinical data on the investigational product(s) that are relevant to the study of the product(s) in human subjects. If a trial is conducted by a team of individuals at a trial site, the investigator is the responsible leader of. Provides up to date safety data obtained during product development;. You should also read this: Rainforest Brochure

ICH GCP - Expectations of stakeholders in the conduct of clinical trials; The investigator’s brochure (ib) is a compilation of the clinical and nonclinical data on the investigational product(s)1 that are relevant to the study of the product(s) in human participants. If you’ve begun combing through the new ich gcp e6(r3) guidelines to understand how the updates will affect your clinical research practices. You should also read this: Brochure Writing For Students

PPT GOOD CLINICAL PRACTICE (GCP) PowerPoint Presentation, free - If you’ve begun combing through the new ich gcp e6(r3) guidelines to understand how the updates will affect your clinical research practices and feel just a little lost. If a trial is conducted by a team of individuals at a trial site, the investigator is the responsible leader of. This training is based on the ich e6 (r2) guideline for. You should also read this: Amg Brochure

PPT GOOD CLINICAL PRACTICE (GCP) PowerPoint Presentation, free - The ich guideline for good clinical practice (gcp) establishes an international standard for the design, conduct, recording, and reporting of clinical trials involving human. And ‒included sections for essential documents and. The investigator’s brochure (ib) is a compilation of the clinical and nonclinical data on the investigational product(s) that are relevant to the study of the product(s) in human subjects.. You should also read this: How To Make The Perfect Brochure

Research Guidelines Research Governance ppt download - Standard for the conduct of trials that involve human participants. Define ich good clinical practice (gcp). The ich guideline for good clinical practice (gcp) establishes an international standard for the design, conduct, recording, and reporting of clinical trials involving human. The investigator’s brochure (ib) is a compilation of the clinical and nonclinical data on the investigational product(s) that are relevant. You should also read this: Brochure Pages Order